Enriched bicarbonate electrolytes show promise in combatting CO2 and the greenhouse effect
Published 01 September, 2021
CO2ER, the electrocatalytic reduction of carbon dioxide (CO2), offers an ideal route for mitigating the greenhouse effect and achieving green CO2 cycling. In recent years, most studies on CO2ER have focused on the intrinsic performance of the catalysts.
While bicarbonate - an electrolyte or negatively-charged ion that is used by the body to help maintain its pH balance - is commonly used in CO2ER, a group of researchers from China set out to explore its role and behaviour in more detail. Their results have been published in the KeAi journal Fundamental Research.
According to the paper’s corresponding author, Jinlong Gong, a professor in the School of Chemical Engineering and Technology at Tianjin University: “Bicarbonate, as a common electrolyte anion, can enhance CO2ER activity via its rapid equilibrium exchange with dissolved CO2. We found that higher concentrations of bicarbonate in the electrolyte provided higher energy efficiency.”
Dr. Wanyu Deng, the lead author of the paper, adds: “The new knowledge gained in this work guides the direct electroreduction of bicarbonate and provides valuable insights into CO2ER with high-concentration electrolytes. This finding is expected to help save abundant energy in the CO2ER process and can pave the way for advancing the progress of CO2ER towards commercialisation.”
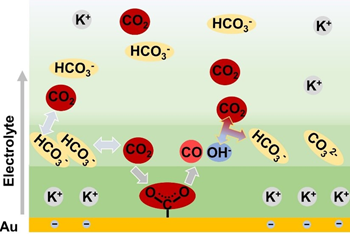
Through in-situ experimental observations, the team discovered an accumulation of negatively-charged bicarbonate anions in the vicinity of the electrode surface. The enriched bicarbonate anions converted to CO2 to provide reactants. In addition, the local concentration of bicarbonate anions was controlled by both the bulk concentration of bicarbonate electrolyte and the cathodic potential. This discovery helped to explain the dependency of CO2ER activity on the local bicarbonate concentration, where the limited cathodic potential caused a plateau in the CO2ER activity.
Commenting on the plateau, Professor Gong says: “This hinders the use of high-concentration electrolytes to enhance activity. Further research is required to systematically explore the intrinsic relationship between CO2ER activity and bicarbonate concentration to achieve better CO2ER performance.”
Contact the corresponding author: Jinlong Gong, jlgong@tju.edu.cn

