Zebrafish usp3 loss promotes hypoxic tolerance
Published 04 February, 2024
Oxygen is an essential element for animal survival. Ocean warming, circadian rhythm, eutrophication, high-density aquaculture, power failures and long-distance live animal transportation can all lead to low oxygen levels in water. This reduction in oxygen can affect the health of aquatic animals, potentially leading and consequent ecological damage or economic loss.
The concentration of dissolved oxygen in water significantly impacts the survival, development, growth and population structure of fish. It is a key environmental factor that determines the success of aquaculture. The hypoxia-inducible factor (HIF)-mediated hypoxia signaling pathway plays a pivotal part in influencing hypoxia (insufficient oxygen) adaptation and tolerance. Under hypoxic conditions, the activity of PHDs decreases, leading to the stabilization and accumulation of HIFα proteins. Stabilized HIFα proteins dimerize with HIF1β proteins, translocate to the nucleus, and induce transcription of genes involved in adaptation or tolerance to hypoxia. The factors that influence the hypoxia signaling pathway mainly affect the stability of the HIFα protein. Hence, identifying and analyzing the regulatory factors of hypoxia signaling pathway will help us understand the mechanism of hypoxia adaptation and hypoxia tolerance in fish.
Recently, a team of researchers in China found that the lack of deubiquitinases usp3 significantly enhanced the hypoxic capacity of zebrafish, and revealed the mechanism of usp3 through inhibiting the hypoxic signaling pathway and hypoxic tolerance in zebrafish.
“We found that the absence of ubiquitin-specific protease 3 (usp3) in zebrafish enhances hypoxia tolerance,” shares Wuhan Xiao, co-corresponding author of the study. “Specifically, zebrafish usp3 binds exclusively to HIF-1αa and triggers its proteasomal degradation, a process contingent upon its deubiquitinase activity. This mechanism results in the attenuation of hypoxia signaling during hypoxic conditions.”
Furthermore, usp3 facilitates the deubiquitination of K63-polyubiquitinated HIF-1αa. Endogenous evidence supports that mammalian USP3 mirrors the regulatory role of zebrafish USP3 in modulating the activity of HIF-1α.
The team’s findings, published in the KeAi journal Water Biology and Security, revealed a novel function for usp3 in influencing hypoxia signaling and showed that tusp3-mediated HIF-1α degradation impairs hypoxia signaling, leading to a decrease in hypoxia tolerance.
Image:
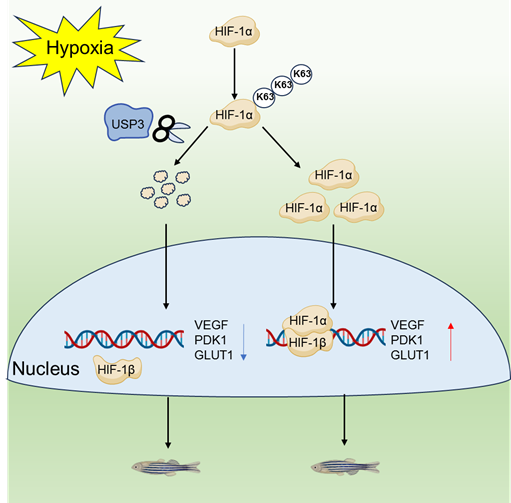
HIF-1α can stabilize its protein through K63-linked polyubiquitination under hypoxia, stabilized HIF1α proteins dimerize with HIF1β proteins, translocate to the nucleus, and induce transcription of genes involved in adaptation or tolerance to hypoxia. USP3 deubiquitinates K63-linked polyubiquitination of HIF-1α to induce HIF-1α degradation and suppress hypoxia signaling.
Contact author name, affiliation, email address: Jun Li & Jing Wang, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, 430072, China, lijun@ihb.ac.cn; wangjing@ihb.ac.cn
Funder: NSFC [31721005 and 31830101 to W. X; 31972786 to J. W.]; The Strategic Priority Research Program of the Chinese Academy of Sciences [XDA24010308 to W. X.]. Funding for open access charge: NSFC [31830101 to W. X.].
See the article: https://doi.org/10.1016/j.watbs.2024.100245