New LiMn2O4 electrode could pave the way for electrochemical recovery of lithium from brine
已发布 25 二月, 2022
Lithium has been dubbed the ‘energy metal of the 21st century’. But with a growing demand for lithium to power electronic products and new energy vehicles, finding sufficient ore resources has proved a challenge.
In fact, most of the world's lithium is now extracted from oil well brines, salt-lake brines and geothermal waters, where it is found in significant qualities. These brine and seawater sources are also typically less expensive to recover.
However, increasing the efficiency of the brine lithium recovery process remains a key goal for researchers working in the field. Electrochemical lithium recovery technology, with its high selectivity, environmental friendliness and low energy consumption, is considered one of the most promising routes to large-scale industrial application. The key challenge is to find an effective electrode material to improve the adsorption capacity of lithium ions and enhance the cycle stability in low energy consumption conditions.
In a paper published in the KeAi journal Green Energy & Environment, researchers in China describe a new approach using a specially-designed version of a LiMn2O4 electrode, which has the potential to address this issue.
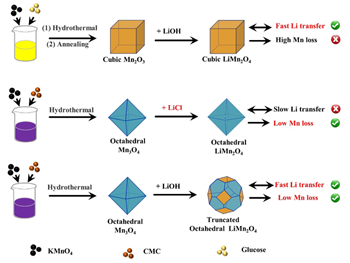
Wenshuai Zhu, one of the paper’s authors and a Professor at China’s Jiangsu University, explains: “The LiMn2O4 electrode is one of the most studied electrode materials in the electrochemical lithium recovery process. However, conventional LiMn2O4 electrodes cannot obtain high cycle stability and high adsorption capacity simultaneously. We found that this is because the (111) crystal plane, which can maintain high cycle stability, has a low lithium adsorption capacity, while the (100) crystal plane, which has high adsorption capacity, has poor cycle stability.”
To solve this problem, Professor Zhu and his group constructed a unique, truncated, octahedral LiMn2O4 electrode that manipulates the synthesis conditions to combine the advantages of the (111) and (100) crystal faces.
Professor Zhu adds: “80% of the global terrestrial lithium resources are liquid state lithium resources in brine. We believe the electrode we have developed could pave the way for electrochemical recovery of that lithium.”
###
Contact the author: Wenshuai Zhu, zhuws@ujs.edu.cn