Expression patterns, features and roles of SRPX2 in disease pathogenesis
Published 07 September, 2025
SRPX2 (Sushi Repeat-Containing Protein X-Linked 2) is a multifunctional regulatory protein that has recently been implicated in various pathological conditions. In a recent review published in Glycoscience & Therapy, a team of Chinese researchers examined the molecular mechanisms by which SRPX2 plays a role in cancer progression, neurological disorders, and fibrosis.
- Expression patterns and structural features of SRPX2
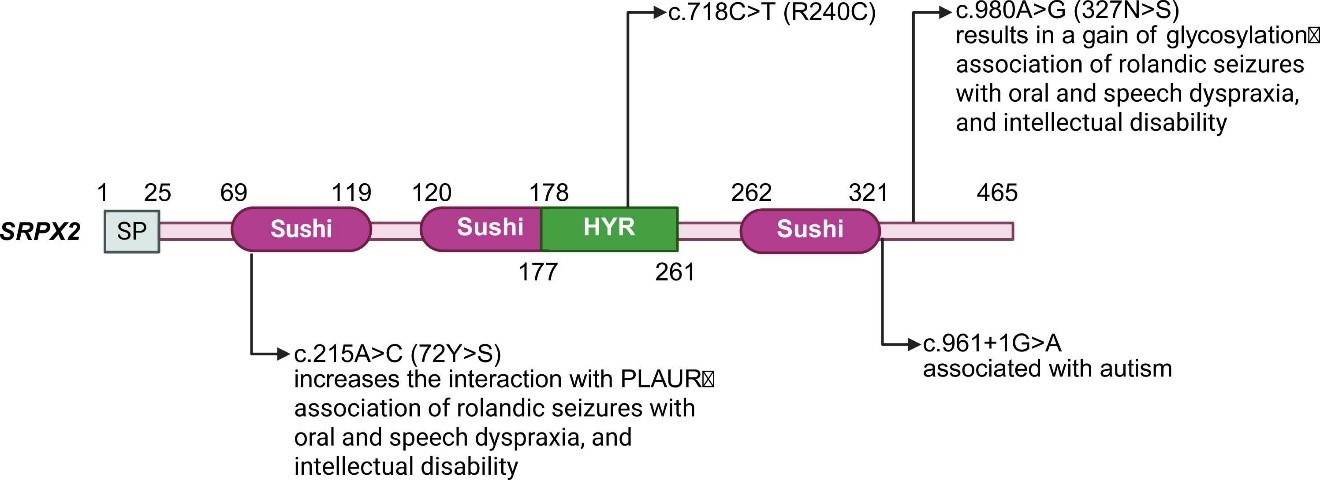
SRPX2, initially identified as SRPUL (sushi-repeat protein upregulated in leukemia) in 1999, is an X-chromosomal gene (Xp22.1) widely expressed in human and mouse tissues, with prominent neuronal expression in brain regions like the Rolandic cortex, suggesting roles in neurodevelopment and language processing. Structurally, it belongs to the sushi repeat protein family, featuring three sushi/CCP domains and one HYR domain. Mutations in SRPX2 (e.g., p.Tyr72Ser, p.Asn327Ser) are controversially linked to neurodevelopmental disorders like Rolandic epilepsy, intellectual disability, and speech dyspraxia, though pathogenicity is debated—especially for p.Asn327Ser, which shows population frequency and potential epistasis with GRIN2A mutations. These mutations disrupt glycosylation and folding, impairing neuronal function. SRPX2 is a novel chondroitin sulfate proteoglycan (CSPG) whose glycosylation modulates stability, interactions, and function. It binds hepatocyte growth factor (HGF), enhancing angiogenesis, and interacts with FOXP2, implicating it in synaptic and vocalization pathways. Despite these insights, its definitive mechanistic roles in disease remain unclear.
- Potential role of SRPX2 in diseases
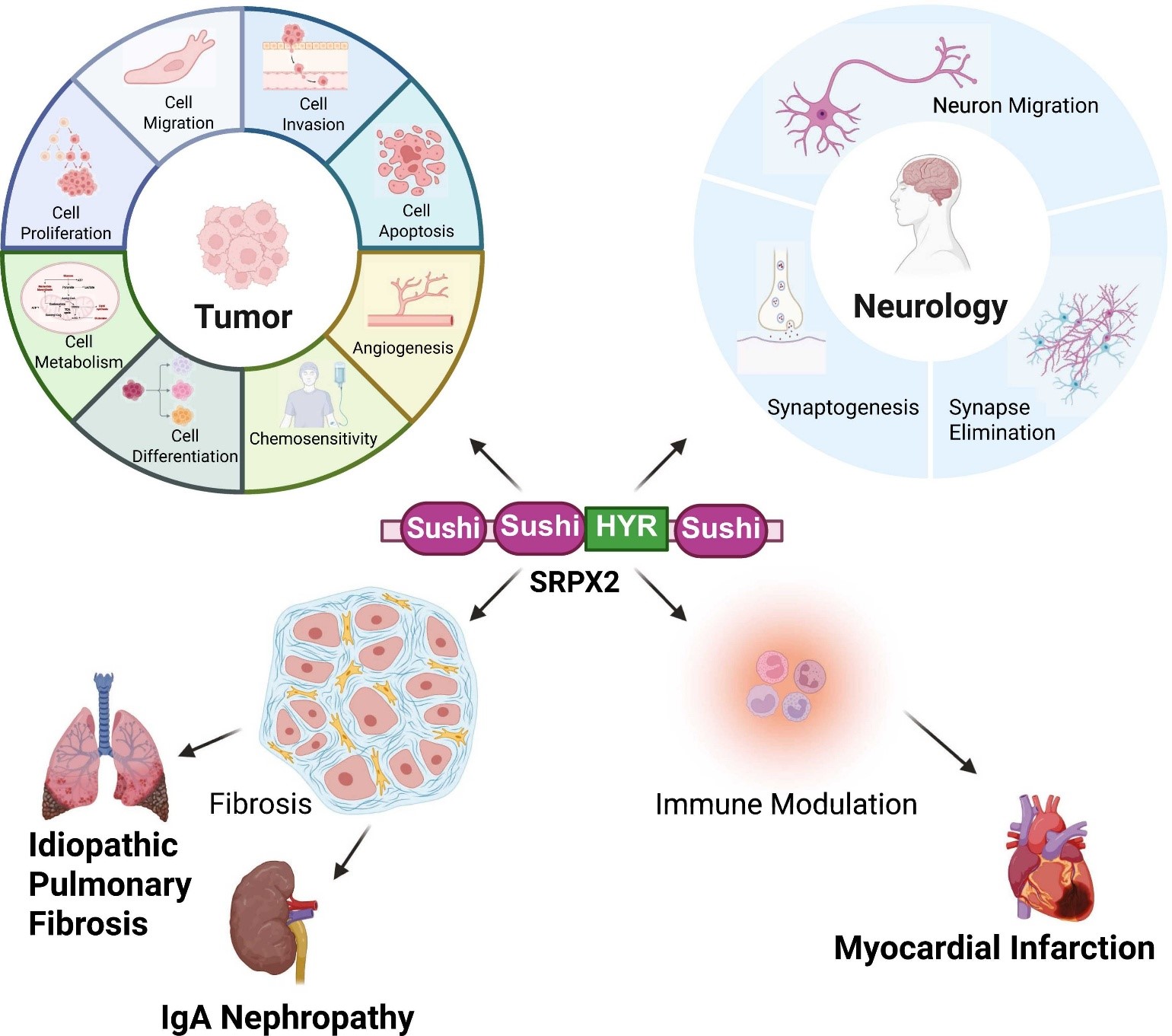
SRPX2 plays multifaceted and significant roles across a wide spectrum of human diseases, primarily acting as a key regulatory molecule influencing critical cellular pathways.
Cancer: SRPX2 is recognized as an oncogenic driver. Its overexpression is associated with numerous cancers (gastric, pancreatic, thyroid [PTC & ATC], colorectal, osteosarcoma, esophageal squamous cell carcinoma, oral squamous cell carcinoma, glioblastoma, acute myeloid leukemia, skin melanoma). It promotes tumor progression by enhancing cell proliferation, migration, invasion, metastasis, angiogenesis, and therapy resistance (chemotherapy, immunotherapy evasion). It achieves this by dysregulating crucial signaling pathways, including TGF-β, PI3K/AKT/mTOR, Wnt/β-catenin, Hippo/YAP, FAK/SRC/ERK, and interacting with μPAR. High SRPX2 expression is strongly linked to poor prognosis and increased invasiveness across these cancers, making it a valuable prognostic biomarker and therapeutic target.
Rolandic Epilepsy and Language Disorders: Associated with bilateral perisylvian polymicrogyria, epilepsy, speech difficulties (dyspraxia), and intellectual disability. It functions within the FOXP2-SRPX2/μPAR pathway and interacts with GRIN2A (NMDA receptor), regulating language-related synaptic plasticity and cortical development.
Autism Spectrum Disorder (ASD): Genetic variations (e.g., splice site mutation) and animal models link SRPX2 deficiency to social deficits and communication abnormalities, potentially via disrupted synaptic density and dysregulated complement-mediated synaptic pruning via interaction with C1q.
Traumatic Brain Injury (TBI): Acute decreases in SRPX2 levels post-TBI may indicate hypothalamic-pituitary axis dysfunction, suggesting its potential as an early diagnostic biomarker.
Idiopathic Pulmonary Fibrosis (IPF): SRPX2 is overexpressed and promotes fibrosis via a TGF-β1/SMAD3/SRPX2 positive feedback loop, driving fibroblast-to-myofibroblast transition and collagen deposition. Silencing SRPX2 shows therapeutic potential.
Myocardial Infarction (MI): SRPX2 expression is decreased after I/R injury. It exhibits cardioprotective effects by reducing apoptosis and ER stress, potentially via inhibiting the PI3K/Akt/mTOR pathway.
Angiogenesis: SRPX2 acts as a pro-angiogenic factor by interacting with μPAR and integrin αvβ3, activating PI3K/Akt and Ras/MAPK/FAK pathways to promote endothelial cell migration and sprouting.
Embryonic Stem Cell Differentiation: Acts as a downstream target of NFATc3 and c-JUN, regulating lineage differentiation and epithelial-mesenchymal transition (EMT) in human embryonic stem cells.
IgA Nephropathy (IgAN): Identified as a key secretory gene associated with IgAN, potentially linked to tubulointerstitial fibrosis.
Diabetic Peripheral Neuropathy (DPN): Bioinformatics identifies SRPX2 as a significant hub gene, potentially involved in inflammation, ECM remodeling, and immune modulation related to DPN pathogenesis.
- Conclusion
SRPX2 emerges as a critical molecule with diverse functions, acting as an oncogene in numerous cancers, a key regulator in neurodevelopmental and neurological disorders, and a significant player in fibrosis, cardiovascular injury, angiogenesis, and other conditions. This makes SRPX2 a highly relevant biomarker for prognosis and diagnosis and a promising target for therapeutic intervention across multiple disease areas. It requires future studies that integrate structural biology with computational drug design. This multidisciplinary approach is essential for addressing current challenges and achieving precise modulation of SRPX2 activity in clinical environments.
Contact author details:
Qifei Cong, Institute of Neuroscience, Soochow University, Suzhou, Jiangsu, China, 215123. qfcong@suda.edu.cn
Funder: This work was supported by grants from the National Natural Science Foundation of China (NO. 32200778, 32471014), Natural Science Foundation of Jiangsu Province (NO. BK20220494), Suzhou Medical and Health Technology Innovation Project (NO. SKY2022107), the Clinical Research Center of Neurological Disease in The Second Affiliated Hospital of Soochow University (NO. ND2022A04), State Key Laboratory of Drug Research (NO. SKLDR-2023-KF-05), Jiangsu Shuangchuang Program for Doctor, Young Science Talents Promotion Project of Jiangsu Science and Technology Association (TJ-2023-019), Suzhou International Joint Laboratory for Diagnosis and Treatment of Brain Diseases, the Priority Academic Program Development of Jiangsu Higher Education Institutes (PAPD), the Project of MOE Key Laboratory of Geriatric Diseases and Immunology (No. KJS2501) to Qifei Cong; Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYCX23_3235) to Jiali Wang.
Conflict of interest: The authors declare no competing financial interests.
See the article: https://doi.org/10.1016/j.glycos.2025.100005