Ammonia induction strategy for preparation of transition metal oxides / zeolite H2S adsorbent
Published 19 March, 2024
Blast furnace gas (BFG) is an important by-product energy for the iron and steel industry and has been widely used for heating and electricity generation. However, the undesirable contaminants, such as COS, CS2 and H2S, in BFG generate harmful environmental emissions. The desulfurization of BFG is urgent for integrated steel plants due to the stringent ultra-low emission standards. Compared with other desulfurization materials, zeolite-based adsorbents represent a viable option with low costs and long service life. However, the sulfur capacity of zeolite is relatively low and needs to improve.
Impregnation of transition metal oxides onto zeolite is a common strategy to prepare H2S adsorbent. However, this method usually results in the agglomeration of metal particles during calcination, forming relatively large metal particles. The large metal particle may increase the gas diffusion resistance in adsorbent and inhibit the desulfurization performance. Therefore, minimizing the metal particles on zeolite with a high loading is the key for the preparation of an adsorbent with high sulfur capacity.
To this end, a team of researchers from the Institute of Process Engineering, Chinese Academy of Sciences, has proposed an ammonia induction strategy. In the process of loading copper oxide onto 13X zeolite by impregnation method, ammonia was introduced, and a Cu based complex formed firstly and then adsorbed on zeolite, which was converted to CuO in the subsequent calcination process.
"The introduction of ammonia effectively inhibits the agglomeration and increases the dispersibility of CuO particles during calcination, prevents the plugging of zeolite pores, improves the diffusion of H2S during desulfurization, and thus increases the adsorption rate and sulfur capacity of H2S adsorbent." shared Erping Cao, lead author of the study published in the KeAi journal Green Energy and Environment. "The H2S adsorption capacity of NH3-CuO/13X adsorbent prepared by ammonia induction is more than twice that of CuO/13X adsorbent".
Notably, similar results were obtained when the ammonia induction strategy was applied to the other kind of zeolite-based adsorbents.
"Based on the ammonia induction strategy, we have provided a general approach for the preparation of transition metal oxide/zeolite adsorbents with high sulfur capacity," adds corresponding author Yanbin Cui.
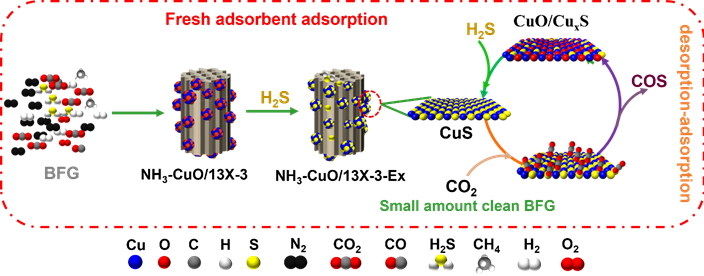
Image: H2S removal and regeneration mechanism of NH3–CuO/13X-3 adsorbent. CREDIT: The AUTHORS
Contact author details:
Yanbin Cui, State Key Laboratory of Mesoscience and Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China,
E-mail: ybcui@ipe.ac.cn
Funder:
This study is financially supported by National Natural Science Foundation of China (Grant. 22076189), National Key Research and Development Program of China (No. 2023YFC3707003), and the Joint Fund of Yulin University and Dalian National Laboratory for Clean Energy (Grant. YLU-DNL Fund 2022003).
Conflict of interest:
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
See the article:
Cao, E. P., et al., Ammonia-induced CuO/13X for H2S removal from simulated blast furnace gas at low temperature. Green Energy & Environment, doi.org/10.1016/j.gee.2024.02.002.

