Assessing the potential of genotoxicity and phototoxicity of triazine UV filters
Published 22 July, 2025
While moderate sun exposure benefits health, excessive ultraviolet (UV) radiation can lead to sunburn, photoaging and long-term risks like skin cancer. Triazine-based UV filters, including ethylhexyl triazone (EHT) and diethylhexyl butamido triazone (HEB), are sued for their broad-spectrum absorption (280–380 nm), high molecular weight, strong photostability and low skin penetration.
Nonetheless, alhough EHT is approved in the EU (max 5%) and several Asian markets, it remains unapproved in the U.S., reflecting differing regulatory frameworks. Safety assessments now rely heavily on validated in vitro methods, such as the bacterial reverse mutation, chromosomal aberration, and micronucleus assays, to address mutagenicity and chromosomal damage without animal testing.
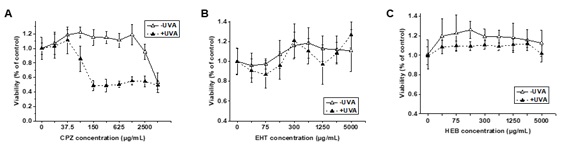
To that end, a team of researchers from China and Malaysia evaluated the safety profiles of EHT and HEB through integrated genotoxicity and phototoxicity assessments. The researchers employed OECD-validated testing strategies — bacterial reverse mutation (Ames test), chromosomal aberration, and micronucleus assays — to assess mutagenic, clastogenic, and aneugenic risks in vitro, while phototoxicity was evaluated via 3T3 neutral red uptake (NRU) assays and in vivo guinea pig models.
“We did not observe any genotoxic effects; Ames test showed no significant increase in revertant colonies across five bacterial strains, while chromosomal aberration assays revealed zero abnormalities in mammalian cells, and micronucleus frequencies remained negligible at concentrations up to 1 mg/mL,” shares corresponding author Yibei Zhan.
Furthermore, even at 5 mg/mL—which exceeds typical cosmetic use levels—only minor micronucleus increases occurred, deemed biologically irrelevant compared to positive controls.
“Phototoxicity assessments further confirmed safety,” adds Zhan. “Both filters exhibited photo-irritation factors of less than 2, and mean photo effects of lower than 0.1 in the 3T3 NRU assay, while guinea pig tests showed complete absence of erythema or edema post-UV exposure.”
The consistent absence of toxicity across all endpoints—attributed to the compounds' stable triazine architectures—provides evidence supporting their safety for topical applications.
“Our multi-assay approach resolves lingering doubts about these UV filters, particularly for regions like the U.S. and Asia where regulatory approval requires extensive safety data,” says Zhan.
The team’s findings, published in the Journal of Dermatologic Science and Cosmetic Technology, not only validate current EU approvals but also offer critical experimental transparency to accelerate global regulatory acceptance, potentially replacing less stable alternatives in next-generation sunscreens.
The authors recommend future work explore oxidative stress markers and human metabolic pathways using advanced 3D tissue models.
Contact author name: Yibei Zhan, College of Chemistry and Chemical Engineering, Hubei Polytechnic University, Huangshi, Hubei, ybzhan@hbpu.edu.cn
Funder: This work was supported by the National Natural Science Foundation of China (No. 81600459), National Natural Science Foundation of Hubei Province (No. 2016CFB312).
Conflict of interest: The authors declare no conflicts of interest.
See the article: Wei Y, et al. Assessment of the potential genotoxicity and phototoxicity of triazine UV filters. J Dermatol Sci Cosmet Technol. 2025; 2(2): 100087. https://doi.org/10.1016/j.jdsct.2025.100087