New 3D, self-supported electrode could pave the way for large-scale hydrogen production
Published 02 December, 2021
Hydrogen energy is considered one of the most promising sources of clean energy for the 21st century. Not only does it have the highest calorific value (volume of heat generated per consumed unit), it also produces almost zero pollution. Electrocatalytic water splitting is currently one of the most effective methods to obtain hydrogen efficiently, due to its high energy use rate and simple operation. The key challenge is to find an efficient catalyst to increase the effectiveness of the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) processes. The goal of this catalyst is to reduce the overpotential, in other words, any kind of loss or non-ideal reactions during HER and OER.
Researchers at the Institute of High Energy Physics (Chinese Academy of Sciences) and Ningbo Institute of Materials Technology and Engineering (Chinese Academy of Sciences) have developed a 3D, self-supported electrode that reduces the overpotential in the water splitting process. In their recent paper, published in the KeAi journal Green Energy & Environment, they outline their proposed approach, which prepares hierarchical and honeycomb alloy for overall water splitting by direct electrodeposition – a process that assembles solid materials from molecules, ions or complexes in a solution. In this case, that solution is nickel (Ni) foam in high-temperature molten salt.
Professor Weiqun Shi of the Institute of High Energy Physics explains: “Ni-based metallic foams as catalyst carriers are of specific interest due to their large specific surfaces and open cell structures. However, their fabrication is usually conducted by electrodeposition or hydrothermal synthesis in aqueous solution or organic electrolyte, which does not allow the introduction of more reactive metallic elements, such as rare earth elements.”
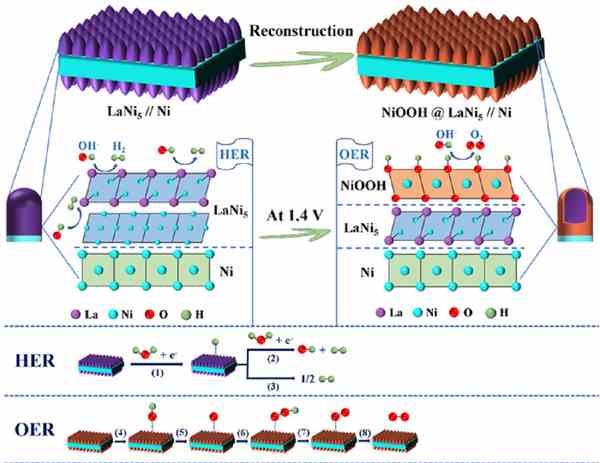
To solve this problem, Professor Shi and his colleagues alloyed the nickel foam and lanthanum – one of the rare earth elements - by electrodeposition in high-temperature molten chloride salt, which produced an alloy containing the lanthanum and nickel.
According to Professor Shi: “The hierarchical alloy has fully-exposed active sites of sub-micron pores, like a 3D, self-supporting and honeycomb network structure, which promotes the rapid electrochemical reaction and gas evolution at the surface of the alloy in the process of hydrogen production.
“On the other hand, due to the numerous submicron channels on the surface of the honeycomb alloy, a large amount of nickel element migrates to the surface and transforms into another highly-active species, which greatly promotes oxygen production.” He adds: "As the method developed in this work can be readily scaled up, it may pave the way for fabricating more efficient, foam, metal-based materials for large-scale hydrogen production.”
###
Contact the author: Weiqun Shi, shiwq@ihep.ac.cn